
The leader in PNS clinical data
StimRouter is the industry leader for peripheral nerve stimulation clinical data. StimRouter has the only published randomized, controlled trial (RCT) for peripheral nerve stimulation targeting multiple nerves.
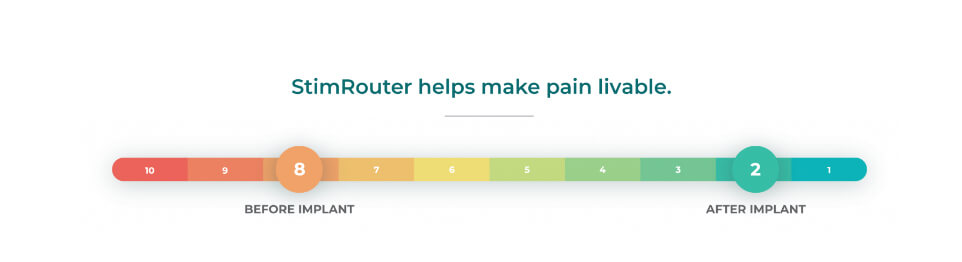
StimRouter provides significant, sustainable pain relief
The StimRouter study for chronic peripheral pain was designed to assess the safety and effectiveness of PNS. Ninety-four subjects with chronic peripheral nerve pain in the upper extremity, lower extremity or trunk, were implanted with the StimRouter Neuromodulation System.3
- 50% of the treatment group rated their satisfaction 8 or higher on a 10-point scale
- 53% of the treatment group rated their global impression of change in activity limitation, symptoms, emotions and overall quality of life-related to their painful condition between 5 and 7 on a 7-point scale
- 31% of the treatment group rated their satisfaction at a 10 on a 10-point scale
- There were zero infections, zero lead migration, and zero serious adverse events related to StimRouter through 12-month follow-up
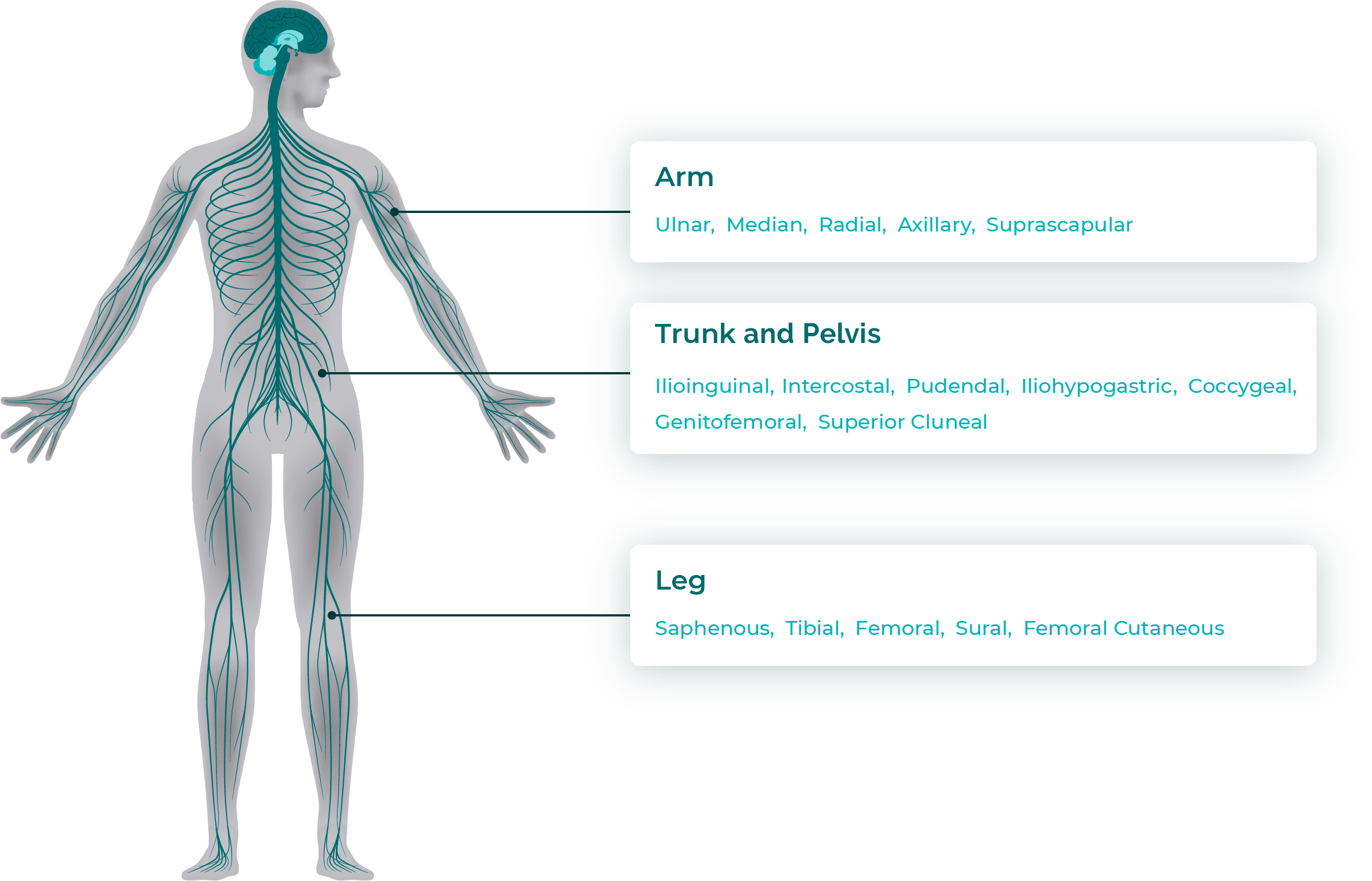
Peripheral nerves targeted in the clinical study
StimRouter can be placed in numerous locations on the arms, trunk, and legs based on patient need. During the StimRouter RCT, the StimRouter was implanted on 19 different nerves.
Reasons for the patient’s chronic pain were varied and included: post-stroke shoulder pain, pain after joint replacement surgery, complex regional pain syndrome (CRPS), diabetic neuropathy, neuroma, entrapment syndromes, intercostal neuralgias, and other peripheral injuries or diseases not of craniofacial origin.

Download the StimRouter PNS Clinical Data eBook

